BACKGROUND: Immunosuppressive therapy (IST) (anti-thymocyte immunoglobulin (ATG) and cyclosporine (CSA)) is the treatment of choice for patients with acquired severe aplastic anemia (SAA) who are ineligible for hematopoietic stem cell transplantation (HSCT), which results in a hematological response (HR) in about 60-70% of SAA patients. TPO is a hepatic-secreted hematopoietic growth factor that specifically regulates platelet production, induces hematopoietic stem cell differentiation to megakaryocytes by binding to the TPO receptor (MPL), stimulates megakaryocyte proliferation and differentiation, and promotes platelet production and release. Several previous studies have shown that IST combined with rhTPO or hetrombopag can improve the early hematologic response of SAA patients, especially the proportion of good hematologic response (good HR) in the early stage. RhTPO and TPORA have different binding sites with thrombopoietin receptor (c-MPL) and do not have competitive binding, which may produce synergistic effect. A retrospective study showed that eltrombopag combined with rhTPO increased the IST HR and improved the quality of hematologic remission in SAA with mild adverse effects. Therefore the combination of TPORA and rhTPO in SAA patients undergoing IST treatment may result in faster and higher HR rates. Clinical studies have confirmed that hetrombopag, act similarly to eltrombopag in SAA, but there are no clinical studies of hetrombopag in combination with rhTPO in SAA.
OBJECTIVE: To evaluate the early-efficacy and safety profile of IST in combination with hetrombopag, and rhTPO in patients with primary, severe aplastic anemia.
METHODS: This study was designed for patients with primary aplastic anemia major aged 14-70 years after January 2023 from a single center. This group were planned to be get treat of IST combination with rhTPO 15000U/d for 8 weeks from the first day of ATG and hetrombopag 15mg/d for at least 6 months. The primary endpoint was to observe the good response rate, blood product transfusion and safety profile of the study regimen for the treatment of primary SAA for 3 months.
Efficacy Criteria:Complete response (CR): HGB>100 g/L, PLT> 100×10 9/L and ANC>1.5×10 9/L; ② Good partial response (GPR): HGB>80g/L, PLT> 50×10 9/L and ANC>1.0×10 9/L; ③ Partial response (PR): withdrawal from blood product transfusion dependence (HGB>70g/L, PLT> 20×10 9/L), hematologic tests improved and no longer met the SAA criteria, but blood count did not meet the GPR criteria. ④No response to treatment (NR): Continuous transfusion dependency was classified as no response. and/or blood count still met SAA criteria. We defined CR+GPR+PR as HR and CR+GPR as good HR.
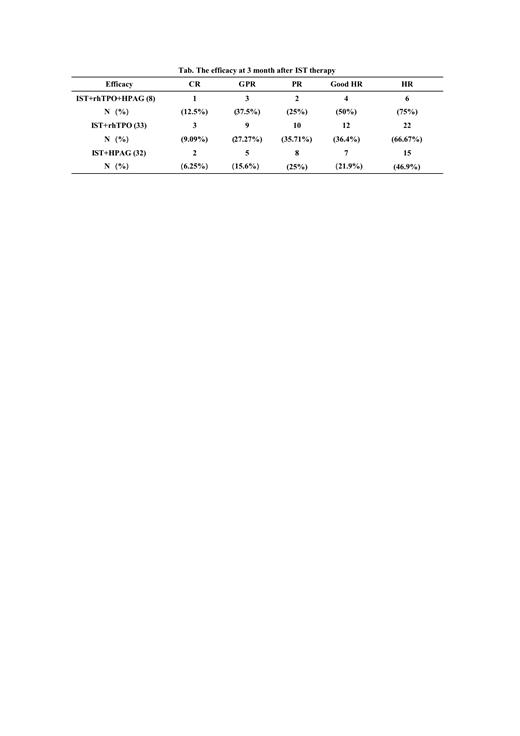
RESULTS: The trial group proposes to enroll 39 patients and currently includes 8 patients who can be evaluated for 3-month efficacy of IST treatment. And the control group includes two groups of IST combined rhTPO group and IST combined HPAG group in our previous center, and the cohort study of the results of the three groups. In this study, the HR rate of 8 patients can be assessed at present as 75% (6/8), and the rate of good HR was 50% (4/8). The HR of 33 patients in the control IST+rhTPO group was 66.67% (22/33), and the rate of good HR was 36.4% (12/33); the HR rate of 32 patients in the control IST+HPAG group was 46.9% (15/32), and the rate of good HR was 21.9%. The HR rate and good HR rate of the trial group were higher than those of the IST+rhTPO group and the IST+HPAG group (75%v66.67%v46.9%; 50%v36.4%v21.9%). None of the 8 patients enrolled in the trial group had any transformation of myelofibrosis, myelodysplastic syndrome and acute myeloid leukemia during the follow-up period.
CONCLUSION: HR was obtained at 3 months in 6 of the 8 patients with severe remodeling IST+rhTPO+ hetrombopag in this study, with a good HR rate of 50%, which is the highest good efficacy response we know of in a prospective study of Chinese SAA patients to date. This confirms that rhTPO combined with hetrombopag can synergize the recovery of bone marrow hematopoietic function in SAA patients treated with IST, and its clinical effect is superior to that of various TPO-Ras or rhTPO that have been widely used. It is expected that more cases will be included in the later stages and that better results will be obtained.
【Keywords】severe aplastic anemia; recombinant human thrombopoietin; hetrombopag ; immunosuppression treatment.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal